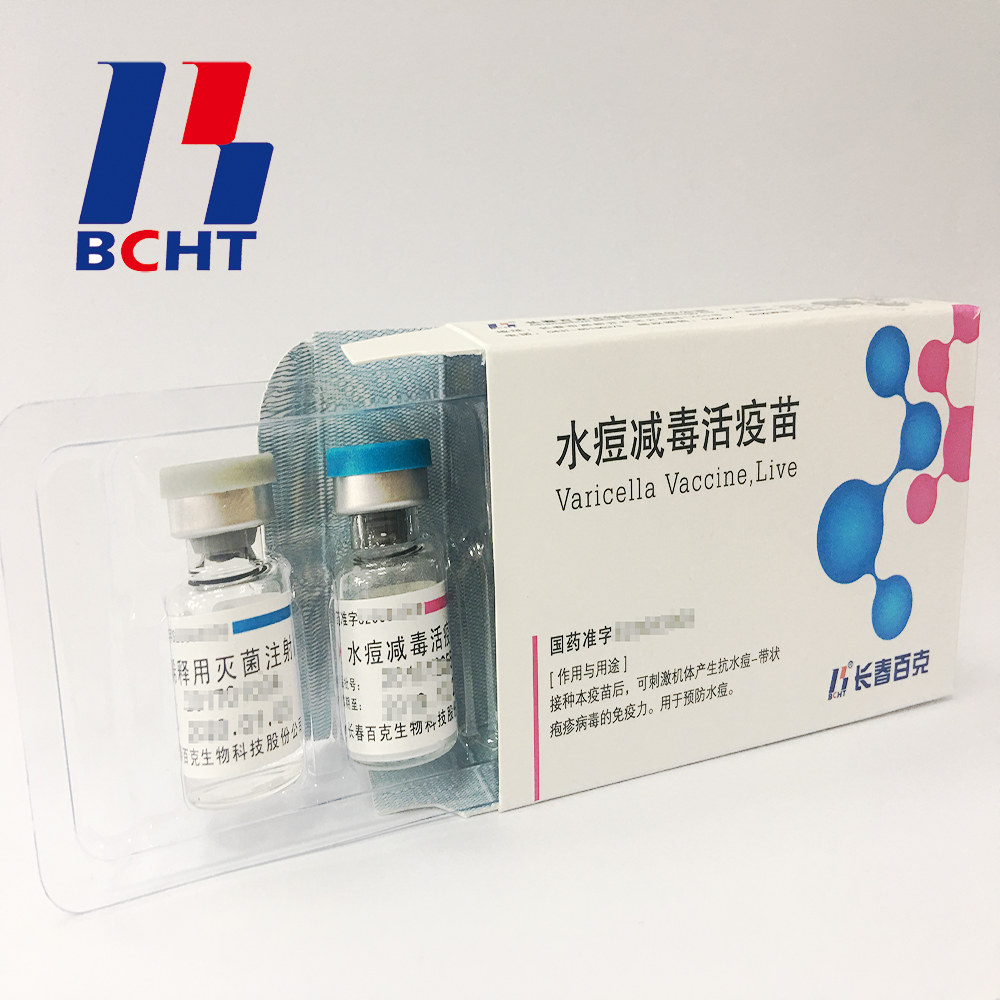
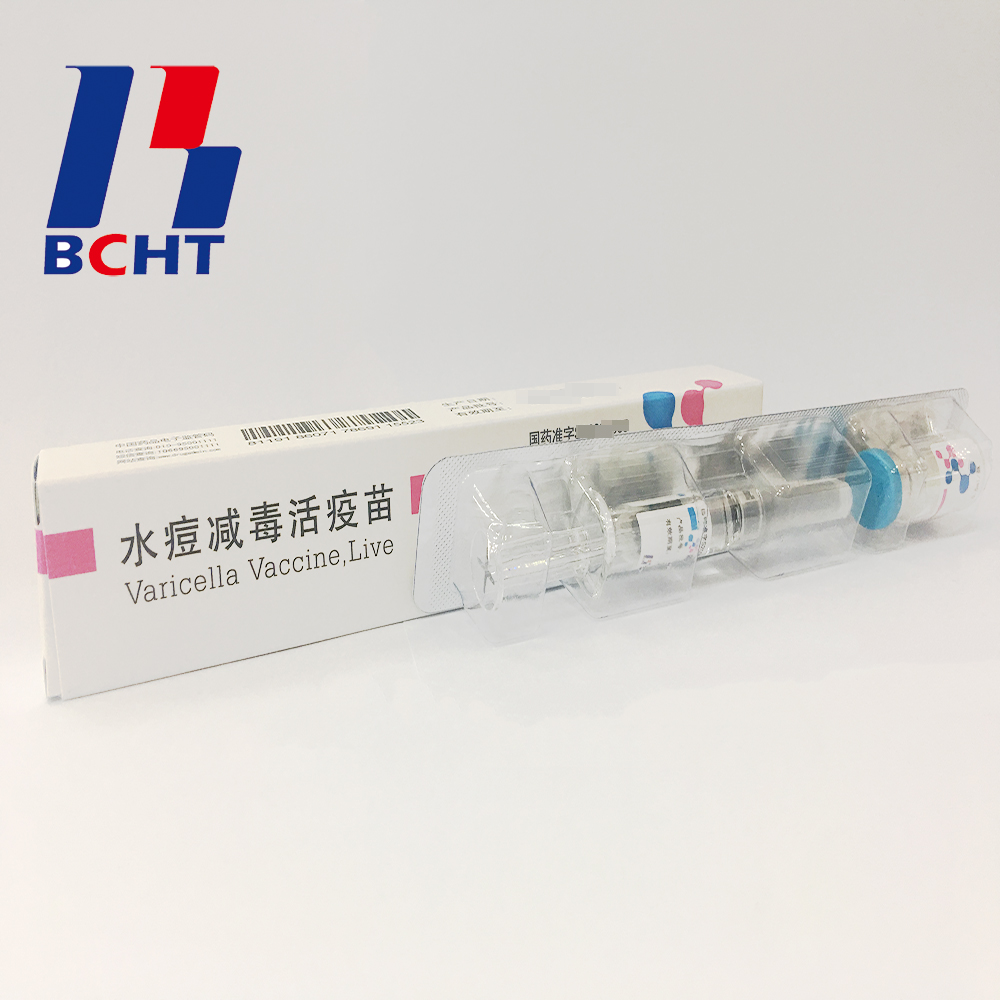
Pfizer is preparing to start a three-in-one immunotherapy combination clinical trial May 11, 2016 Source: US and Chinese medicine source It is reported that Pfizer is preparing to begin the first phase of the first three-in-one immunotherapy combination next year. This combination includes the PD-L1 antibody Avelumab, the 4-1BB agonist Utomilumab, and an OX40 agonist. The trial is said to be ready to detect how much immune activation the human body can tolerate and find the best balance. The annual cost of PD-1 antibodies is now $150,000, so the price of three new immunotherapies is worrying the payment department. Pfizer says pricing can be more flexible because they have all three products. The most dazzling drugs today may be tumor immunotherapy, but the future of cancer treatment belongs to combination therapy. Most people believe that immunotherapy will become the core of future cancer combination therapy, and the recent tumor landing program in the United States has combined immunotherapy as a major direction. There are now a number of immunotherapies combined with chemotherapy, radiotherapy, targeted therapies, combinations of epigenetic therapies, and immunotherapies in clinical trials. Because the gastrointestinal flora seems to have a critical impact on the immune response, a combination of fecal transplantation and immunotherapy is just around the corner. However, the existence of these immunosuppressive mechanisms is justified. If the immune response is out of control, it will have very serious consequences. Autoimmune diseases themselves are a type of disease that seriously affects human health, and immunotherapy has also had serious side effects in clinical trials. The cytokine storm of CAR-T caused several patient deaths. A previous CD28 super agonist called TGN1412 sent all six Phase I clinical volunteers to the intensive care unit. Although PD-1 antibodies are relatively safe, the safety of these combinations is another game. The risk of combining the three new immunotherapies is obvious. Pfizer must be very careful to avoid a repeat of the TGN1412 tragedy. The most successful combination of immunotherapy at present may be the treatment of CTLA-4 antibody Yervoy and PD-1 antibody Opdivo in the treatment of cirrhosis, although the toxicity is very high, but the response rate is indeed much higher than the unilateral. These two combinations are very reasonable in mechanism, CTLA-4 antibody enhances the early immune response, and PD-1 antibody prolongs T cell lethality. Many other combinations lack sufficient evidence in terms of mechanism, and the current preclinical model predicts poor ability, so it can only rely on clinical trials. Nowadays, the classification of patients and the biomarkers that respond to immunotherapy are also in the early stages. These factors make the development cost of combination immunotherapy much higher than that of relatively mature cytotoxic anticancer drugs. Although this kind of speculative trial like today may find important therapies, in the long run, at least some of the above shortcomings need to be supplemented to continue to discover new immunotherapies. Avelumab is estimated to be similar to other PD-1/PD-L1 antibodies, but we know little about 4-1BB and OX40 agonists. Published efficacy and safety information does not show that these two mechanisms have more than PD-1 antibodies. There are certainly some more effective and safe combination therapies between Opdivo and TGN1412, but how to find them is not easy as described above. This has certain similarities with investment stocks. No matter the bull market or the bear market, there are always some stocks that will greatly exceed the market performance, but finding these stocks is a technical activity. Varicella Vaccine Finished Products Finished products of varicella vaccine. It has three qualities,good safety of gelatin-free, long validity period by good stability, better protection with high titer and immune efficacy. These improvements enhanced the vaccine safety and quality, and established BCHT the leading position in varicella vaccine. We have two different packages, penicillin bottle and pre-filled syringe. And it has been exported to other countries, such as India, Philippines. Finished Products Of Rabies Vaccine,Rabies Vaccine For Human Use,Live Biotechnology Chicken Pox,Live Lyophilized Vaccination Changchun BCHT Biotechnology Co. , https://www.ccbcht.net
Pfizer is preparing to start a three-in-one immunotherapy combination clinical trial
Next Article
How to ensure a seed soaking corn seedlings
Prev Article
Peach tree leaf roll control