Culture Plates,Plastic Microplate,Plastic Culture Plates,Culture Plate Microplate Yancheng Rongtai Labware Co.,Ltd , https://www.shtestlab.com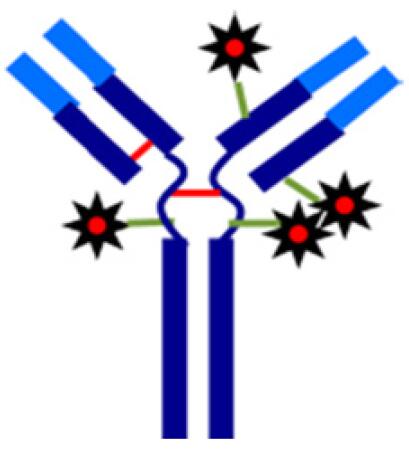
Exactive Plus EMR High Resolution Mass Spectrometer for Analysis of Non-denatured ADCs
Li Jing
Thermo Fisher Scientific (China) Co., Ltd. Chromatography Mass Spectrometry Department
Yang Shujun Han Nian and Jian Li
Shanghai New Concept Biomedical Technology Co., Ltd.
Key words
Exactive Plus EMR; ADCs; cysteine-linked ADCs; Native MS
1 Introduction
Monoclonal antibodies are considered to be highly specific targeted drugs with very high targeting to tumor cells. The antibody-drug conjugates (ADCs) technology is a high-effect chemotherapeutic drug (or small molecule drug) that has an anti-tumor effect on a specific amino acid of an antibody protein to increase the monoclonal antibody. The efficacy of antibodies and the toxicity of small molecule drugs. The production process of ADCs is more complicated than monoclonal antibodies, so in order to ensure the safety and effectiveness of ADCs, the quality of ADCs should be monitored. The drug to antibody ratio (DAR) is an important parameter for evaluating the production process and product quality of ADCs. At present, most of the ADCs in the market and in research ADCs mainly include lysine-linked ADCs coupled based on the lysine of the antibody itself and cysteines based on the reduced inter-disulfide bond of the antibody itself. ADCs (cysteine-linked ADCs) (Figure 1). The DAR determination of two types of ADCs usually has three methods: UV, HIC and MS. Among them, the MS detection method is more and more widely used to determine the DAR of ADCs due to its advantages of fastness, high sensitivity and strong qualitative function. At present, the DAR used by MS for the determination of ADCs is usually based on the traditional RPLC/MS platform. The mobile phase of RPLC under this platform is acidic and contains a high concentration of acetonitrile. The protein is mostly denatured under these conditions, so the measurement results are strict. In the sense, it reflects the DAR after ADCs are degenerated. For lysine-linked ADCs, the above denaturation conditions do not affect the integrity of the antibody structure, so the RPLC/MS platform does not affect its DAR assay. However, for cysteine-linked ADCs, the above denaturing conditions undermine the non-covalent effect of maintaining the spatial structure of the antibody, and the ADCs partially dissociate from their light or heavy chains, so the RPLC/MS platform cannot be used for its DAR assay. With the continuous development and promotion of non-denaturing mass spectrometry (Native MS) technology, the analysis of cysteine-linked ADCs using non-denaturing mass spectrometry shows strong application potential.
The Thermo ScientificTM ExactiveTM Plus EMR mass spectrometer maintains Orbitrap's high resolution, high quality accuracy while extending the mass range to m/z 20000, and improves the transmission efficiency of high quality end ions in hardware design, improving HCD pressure. Make it more suitable for the analysis of intact proteins.
It is suitable for the structural and topological studies of non-denatured proteins and protein complexes with tertiary and quaternary structures and the analysis of cysteine-linked ADCs in non-denaturing conditions in biopharmaceuticals.
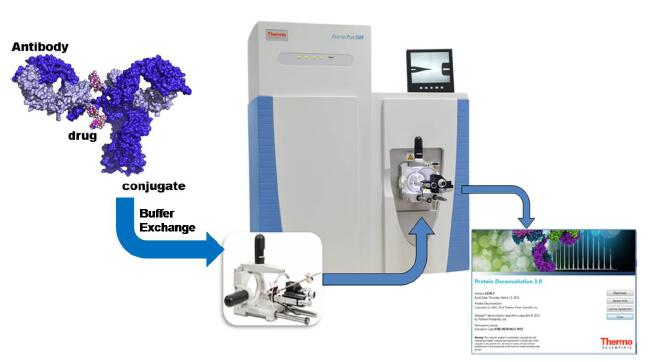
This experiment established a non-denaturing protein analysis process based on ExactiveTM Plus EMR and Protein Deconvolution 3.0 software (Figure 2), and was successfully used in the analysis of cysteine-linked ADCs to determine the accuracy of a mixture of ADCs containing different drug molecules. Molecular weight, and its DAR value was determined.
Figure 1 Schematic diagram of Cysteine-linked ADCs
Figure 2 Analysis flow of non-denatured Cysteine-linked ADCs
2 experimental conditions
2.1 Materials and methods
The sample PCT was Cysteine-linked ADCs. After PNGase F deglycosylation, it was buffer exchanged with Micro Bio-Spin® Chromatography Columns and stored in 100 mM ammonium acetate (pH 7.0) to prepare a solution with a concentration of 5.5 μM. .
2.2 Mass spectrometry
Mass spectrometer
Thermo Scientific Exactive Plus EMR
source of ion
NanoFlex Source with emitter
Mass spectrometry data acquisition mode
Direct Infusion
Ion mode
Positive ions
Spray voltage
1.8 KV
Capillary temperature
275 °C
Capillary temperature
ON
Quality range
m/z 3000–10000
Resolution
17500, 35000, 70,000
S-lens (%)
200
In-souce CID
175
HCD
25
MicroScan
10
Spectra average
50
2.3 Data Processing
Using Thermo Scientific TM Protein Deconvolution 3.0 original deconvoluted spectrum.
The parameters are as follows:
Noise compensation
ON
Minimum adjacent charges
1 to 3
Noise Rejection
95% confidence
3 experimental results
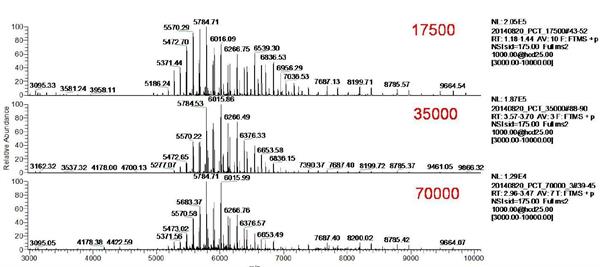
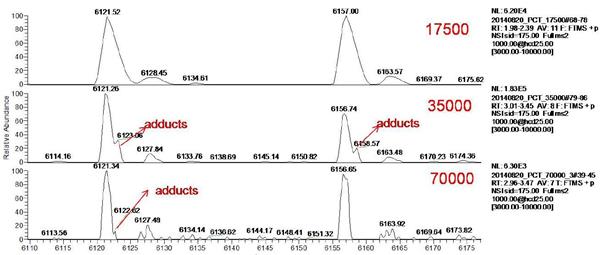
In this experiment, the Orbitrap resolution was set to 17500, 35000, 70,000 by direct injection. The original mass spectrum of ADCs is shown in Figure 3. The mass spectrum peaks of ADCs are mainly distributed in the range of m/z 5000-7000, which is ideal. Signal to noise ratio. Selecting two sets of mass spectral peaks for amplification (shown in Figure 4) reveals that as the mass spectral resolution is gradually increased, the main peak gradually separates from the additive ion peak. The higher the resolution, the more obvious the separation effect.
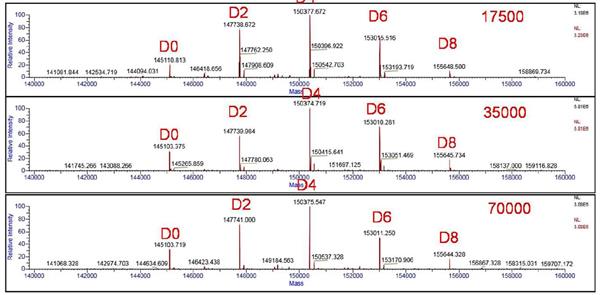
The molecular mass distribution of ADCs at different resolutions after deconvolution by Protein Deconvolution 3.0 software is shown in Figure 5. The mass spectrum will be calculated based on the amino acid sequence of the ADCs and the theoretical molecular weight of the small molecule drug. The peaks are assigned, and we observe from the figure that the five main peaks exhibit an equal mass interval (about 2635 Da), from which it can be inferred that the monoclonal antibody molecules bind different numbers of small drug molecules. Combined with the characteristics of cysteine-linked ADCs, the main peaks were judged to be a mixture of ADCs combining 0, 2, 4, 6, and 8 small molecule drugs. The results were consistent with theoretical predictions.
Figure 3 Original mass spectrum of ADCs for different resolution settings (17500, 35000, 70,000)
Figure 4 Local amplified mass spectrum of ADCs under different resolution settings (17500, 35000, 70,000)
Figure 5 Results of deconvolution of ADCs after different resolution settings (17500, 35000, 70,000)
Resolution
Drug
Load
Measured MW
(Da)
Theoretical MW
(Da)
Delta M
(Da)
17500
D0
145111
145103
7.7
D2
147739
147738
0.3
D4
150378
150374
4.1
D6
153016
153009
6.6
D8
155649
155644
4.4
35000
D0
145103
145103
0.3
D2
147740
147738
1.6
D4
150375
150374
1.1
D6
153010
153009
1.4
D8
155646
155644
1.6
70000
D0
145104
145103
0.6
D2
147741
147738
2.7
D4
150376
150374
1.9
D6
153011
153009
2.4
D8
155644
155644
0.2
Table 1 Accurate molecular weight and mass deviation after convolution at different resolutions (17500, 35000, 70,000)
The exact molecular weights after convolution at different resolutions (17500, 35000, 70,000) are shown in Table 1. It is found that when the resolution is set to 17500, the effective separation of the main peak and the additive ion peak cannot be achieved, quality and theory. The value deviation is large and the maximum deviation is 7.7 Da. When the resolution is gradually increased to 35000, the main peak is gradually separated from the additive ion peak, and the mass deviation is controlled at 0.3-1.6 Da, which has excellent mass accuracy. When the resolution is further increased to 70,000, the separation of the main peak and the additive ion peak is further improved, and the ideal mass accuracy (0.2-2.7 Da) can be obtained to meet the measurement requirements.
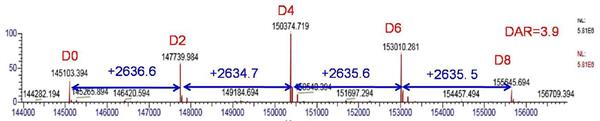
Based on the peak intensity information of the mass spectral peak, the DAR value of the drug can be calculated, which is critical for the effectiveness evaluation of the ADCs. Taking the spectral deconvolution result at a resolution setting of 35000 as an example (as shown in FIG. 6), the DAR value was calculated according to rel = Σ (relative peak area × number of loaded drugs)/100, and the ADCs drug DAR=3.9 was obtained.
Figure 6 Resolution setting of the mass spectrometry of ADCs after deconvolution of the mass spectrum at 35000 (D0-D8 shows a mixture of ADCs carrying different drug molecules)
4 Conclusion
In this paper, the Exactive Plus EMR mass spectrometer, direct injection method, broke through the bottleneck of the traditional RPLC/MS platform can not be analyzed by cysteine-linked ADCs, established the accurate molecular weight determination method of cysteine-linked ADCs, which is the cysteine-linked ADCs monoclonal antibody. Drug discovery and production testing provides an efficient and fast analytical platform. The experimental results show that the Exactive Plus EMR mass spectrometer greatly improves and promotes the identification and analysis of ADCs with its high resolution, ultra-fast scanning speed, high mass accuracy, high sensitivity and extended mass range.
references
1. Albert JR Heck et al. Nat. Methods 2008, 5(4), 927-933.
2. Sara Rosati et al. Nature Protocols 2014, 9(4), 967-976