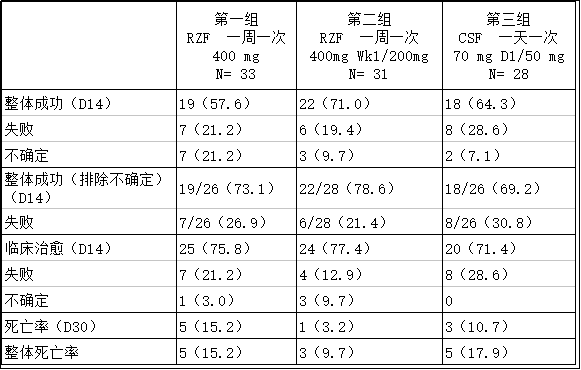
Cidara antifungal drug Rezafungin has positive clinical results March 20, 2018 Source: Sina Pharmaceutical On March 19th, Cidara Therapeutics, a bio company dedicated to the development of new anti-infective diseases, announced the positive top-line research data of the second phase of the trial STRIVE. The trial evaluated the efficacy and safety of the company's main drug candidate rezafungin acetate for the treatment of candidemia and/or invasive Candida infection. The trial reached all major study endpoints, and the safety of intravenous administration once a week. And well tolerated. The data also shows evidence of the effectiveness of rezafungin, which is defined as the ability to remove Candida from blood or other normal sterile sites and to resolve signs associated with infection, and to determine the effectiveness of the method through clinical response and overall survival. Sex. These data allow Cidara to advance rezafungin's next key clinical phase 3 study for the prevention of candidiasis and the prevention of invasive fungal infections. Rezafungin is a new antifungal drug that is being developed as a weekly high exposure therapy for the treatment and prevention of serious invasive fungal infections. Dr. Jeffrey Stein, President and CEO of Cidara, said: "We sincerely thank the participants in the STRIVE research process, the investigators and their field staff for their dedication. This is the first time such an antifungal drug has The potential to be a safe and effective one-week treatment for patients with refractory and fatal invasive Candida infections can allow patients to be discharged early, save money, and improve care. It is particularly encouraging that Rezafungin 400mg is compared to the control drug Caspofungin. The trend toward relative improvement in the treatment of patients treated with the /200mg regimen is consistent. With these data, we can confidently choose a dosing regimen for our upcoming rezafungin Phase 3 clinical trial." The results of the STRIVE trial included efficacy data from 92 treated patients and safety and tolerability outcomes from 104 patients from 31 sites in North America and Europe. The trial did not show statistically superior or non-inferiority, so the efficacy comparison was directional. Efficacy data: For the treatment of Candida/invasive Candida, the STRIVE trial achieved major safety and efficacy indicators. The total efficiency of the rezafungin 400mg/200mg program is the highest. The number of patients with uncertainties in group 1 is high, which is largely one of the reasons for the relative difference between total success rate and clinical cure rate. Safety and tolerance: No trends in adverse events were observed. Rezafungin is well tolerated in both dosing regimens. Treatment of emergency adverse events was observed in most patients, with 88.6% in group 1, 94.4% in group 2, and 81.8% in group 3. The incidence of serious adverse reactions was 37.1%, 27.8%, and 39.4%, respectively. In all study groups, there were 6 adverse events leading to drug discontinuation: the first group of 4, the second group of 1 and the third group of 1 person. Two of the six were considered to be related to the study drug: one group, one group, and three groups of one person. There were two serious adverse reactions that may be associated with the study drug: one in the second group and one in the third group, both patients fully recovered. There were no deaths associated with study drugs, and there were no trends in systemic organ groups or specific adverse events. Dr. George Thompson, associate professor of clinical medicine at the University of California, Davis, said: "There have been no new drugs approved for the treatment of severe candida infection or invasive fungal infections for more than a decade. The results of the STRIVE trial have shown great promise. The therapeutic potential has made rezafungin closer to becoming a potentially new and very practical treatment option, addressing the major limitations of current care standards for patients and clinicians. (Sina Medical Compilation/David) Article Reference Source: Cidara Therapeutics Reports Positive Topline Results from Phase 2 STRIVE Trial of Lead Antifungal Rezafungin Ketone Test Strips,Keto Diastix Test Strips,True Plus Ketone Test Strips,Ketostix Reagent Strips For Urinalysis Changchun LYZ Technology Co., Ltd , https://www.lyzstrips.com
Testing Method:
step 2: Immediately close the container again.
This protects the remaining test strips from humidity and guarantees strip integrity up to the expiry date indicated.
step 3: Dip the test strip for about 1 second into the fresh urine specimen.
Wipe off any excess urine on the rim of the vessel and blot the edge of the test strip on tissue paper.
step 4: Read the result by comparing the test fields with the color scale on the test pack after 30 to 60 seconds.
Cidara antifungal drug Rezafungin has positive clinical results
step 1: Remove a test strip, taking care not to touch the reaction fields.