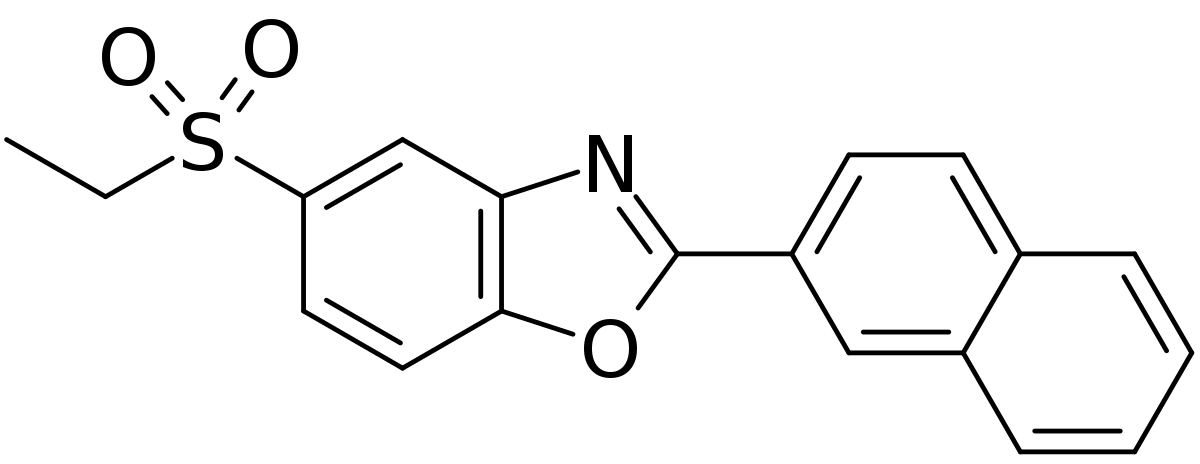
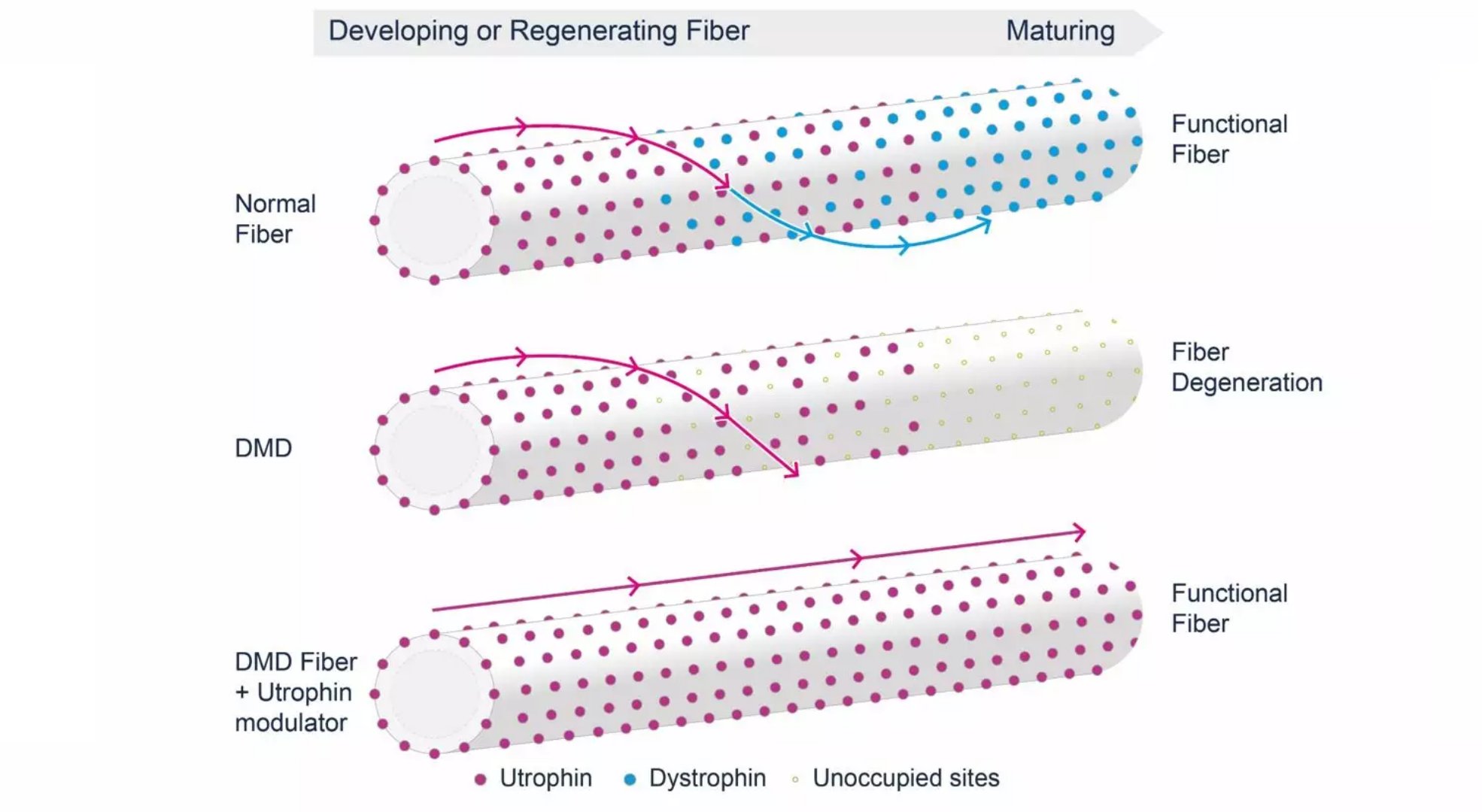
Treatment of Duchenne muscular dystrophy, clinical data of new drug phase 2 is good January 29, 2018 Source: WuXi PharmaTech Summit Therapeutics recently announced a 24-week interim results of Phase 2 clinical trial PhaseOut DMD. Studies have shown that the new drug ezutromid can increase utrophin levels and slow muscle damage. Duchenne muscular dystrophy (DMD) is a genetic disorder caused by the obstruction of muscle cells to produce dystrophin, which maintains the structural and health functions of the muscle. In patients with DMD, the lack of dystrophin leads to a catastrophic cycle of muscle damage and repair. Mustrophin-associated protein (utrophin) has a similar effect to dystrophin in developing and repairing muscle fibers. As muscle fibers mature, utrophin is replaced by dystrophin in healthy individuals. Utrophin and developmental myosin are expressed simultaneously in the early stages of natural muscle repair and then slowly closed. Ezutromid aims to maintain utrophin expression in DMD patients in place of the lack of dystrophin and break this cycle. ▲Ezutromid's molecular structure (Source: Wikipedia) Ezutromid is an oral small molecule drug that is thought to maintain utrophin and slow muscle degeneration. DMD is a rare disease, and the FDA and the European Medicines Agency have issued orphan drug qualifications for ezutromid. In addition, the FDA has issued fast-track qualifications and Rare Pediatric Disease drug qualifications for ezutromid. The PhaseOut DMD Phase 2 clinical trial in DMD patients tested whether ezutromid regulates utrophin levels by measuring muscle fiber regeneration and muscle fat infiltration in utrophin protein and muscle biopsy. The 48-week clinical trial brought together 40 patients in the United Kingdom and the United States. The focus of this interim analysis test showed that ezutromid treatment statistically and significantly reduced muscle damage. In a 24-week muscle biopsy, the average developmental myosin (a biomarker of muscle damage, found in repair fibers) was 23% less than baseline (11.37% to 8.76%, 95% CI, -4.33, -0.90). Fourteen of the 22 patients showed a decrease in developmental myosin, with five of them showing a reduction of more than 40%. The mean utrophin protein level increased by 7% (0.370 to 0.396, 95% CI, -0.005, 0.058) in the 24-week biopsy compared to baseline. In addition, all patients achieved plasma levels of ezutromid sufficient to modulate utrophin. The average muscle fiber diameter decreased from 42.1 microns at baseline to 40.3 microns at 24 weeks. The average fat fraction of the quadriceps (thigh) was 14.7% at baseline and 18.5% at 24 weeks (n = 37). Exploratory testing includes physical function testing of DMD patients that decline over time. Among them, the average six-minute walking distance is 404 meters from the baseline and 395 meters at 24 weeks (n = 39). The North Star Ambulatory Assessment was 25.0 at baseline and 24.4 at 24 weeks (n = 39). Ezutromid has been well tolerated so far. ▲Ezutromid's mechanism of action (Source: Summit Therapeutics official website) “The significant reduction in muscle damage observed in patients at 24 weeks of the PhaseOut DMD trial and the increased expression of utrophin are very encouraging, suggesting that ezutromid can slow the never-ending cycle of muscle fibrosis and regeneration as a DMD marker.†Dubowitz nerve Dr. Francesco Muntoni, Ph.D., director of the Muscle Center and principal investigator of PhaseOut DMD, said: "These favorable interim results have undoubtedly made utrophin regulation an important step in the development of all DMD patient therapies." Dr. Dame Kay E. Davies, co-founder of Summit and professor of anatomy at Oxford University, said: "The constant production of utrophin protein to prevent DMD progression has been well documented in preclinical studies. These data provide the first regulation of utrophin in patients. Evidence. If further research is based on this, they can make ezutromid a universal treatment for disease improvement and bring hope to all patients and families with DMD." PhaseOut DMD is currently in progress and the top line results are expected to be reported in the third quarter of 2018. In addition, Summit plans to conduct a randomized, placebo-controlled trial that will help ezutromid accelerate approval in the US and EU. Additional details of the mid-week data will be presented at future medical and scientific conferences. We hope that this trial will continue smoothly and bring hope to patients and families with this painful disease. Reference materials: [1] Summit Therapeutics unveils encouraged early data for Duchenne drug [2] Ezutromid significant reduced muscle damage in DMD patients in 24-week interim data from summit's PhaseOut DMD clinical trial API Powder (Active Pharmaceutical Ingredient) Active Pharmaceutical Ingredients refer to the raw materials used in the production of various preparations. They are the effective ingredients in the preparations. They are various powders, crystals, extracts, etc., prepared by chemical synthesis, plant extraction or biotechnology, but Substances that the patient cannot take directly.
According to its source,Active Pharmaceutical Ingredients are divided into two categories: synthetic chemical drugs and natural chemical drugs.Chemical synthetic drugs can be divided into inorganic synthetic drugs and organic synthetic drugs. Inorganic synthetic drugs are inorganic compounds (extremely elements), such as aluminum hydroxide and magnesium trisilicate used to treat gastric and duodenal ulcers; organic synthetic drugs are mainly composed of basic organic chemical raw materials, through a series of organic Drugs made by chemical reactions (such as aspirin, chloramphenicol, caffeine, etc.).
Natural chemical drugs can also be divided into biochemical drugs and phytochemical drugs according to their sources.Antibiotics are generally made by microbial fermentation, which belongs to the category of biochemistry.In recent years, many semisynthetic antibiotics are the combination of biosynthesis and chemosynthesis.Among APIs, organic synthetic drugs account for the largest proportion in variety, yield and output value, which is the main pillar of chemical pharmaceutical industry.The quality of API determines the quality of preparation, so its quality standards are very strict. All countries in the world have formulated strict national pharmacopoeia standards and quality control methods for its widely used API
Active Pharmaceutical Ingredient,API powder,minoxidil,monobenzone PYSON Co. ,Ltd. , https://www.pysonbio.com
Treatment of Duchenne muscular dystrophy, clinical data of new drug phase 2 is good