Release date: 2014-08-26 On August 13, South Korea submitted a notice to the WTO to amend the standards and specifications for electromagnetic safety of medical devices to harmonize Korean domestic regulations with international standards. Â Source: China National Times Tetrabromobisphenol A CAS No.79-94-7 Tetrabromobisphenol A Basic Information
Tetrabromobisphenol A Structure
Tetrabromobisphenol A,Tetrabromobisphenol A Msds,Tetrabromobisphenol A (Tbbpa),Tetrabromobisphenol A Bis (2 3-Dibromopropyl Ether),Tetrabromobisphenol A Bis Shandong YingLang Chemical Co.,Ltd , https://www.sdylhgtrade.com
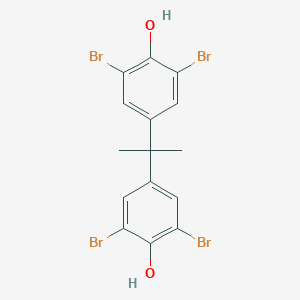
Product Name: Tetrabromobisphenol A
CAS: 79-94-7
MF: C15H12Br4O2
MW: 543.87
EINECS: 201-236-9
Mol File: 79-94-7.mol
Melting point 178-181 °C(lit.)
Boiling point 316 °C
density 2.1
storage temp. 2-8°C
solubility Insoluble
form neat
South Korea plans to revise medical device electromagnetic safety regulations
The international standards applicable to this revision are: IEC 60601-1-2:2007 Medical Electrical Equipment - Part 1-2: General requirements for basic safety and basic performance - Parallel standard: Electromagnetic compatibility - Requirements and testing. CISPR 11:2010 Industrial, Scientific and Medical Equipment - Radio Frequency Interference Characteristics - Limits and Measurement Methods.
Â
The deadline for comments on this notification is 60 days from the date of notification.
Next Article
Qiuqiu six food anti-autumn diet effects vary
Prev Article
How to apply pesticides rationally