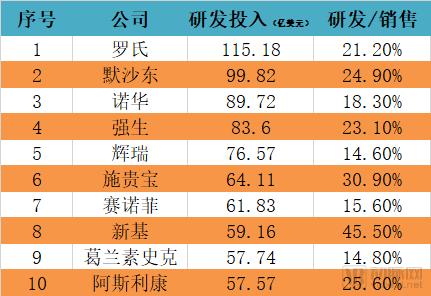
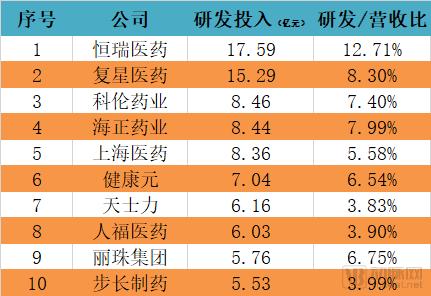
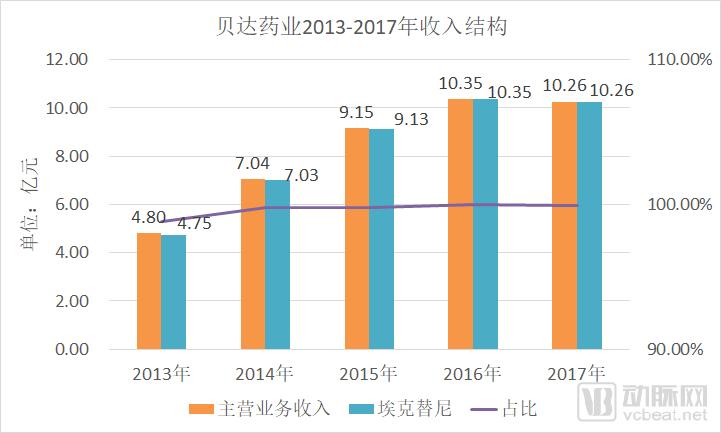
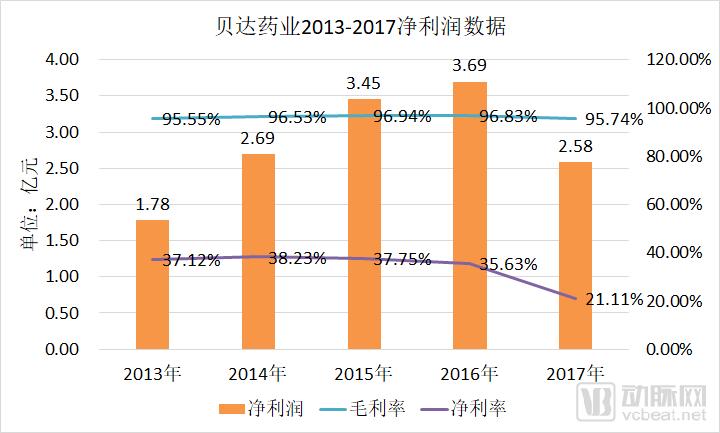
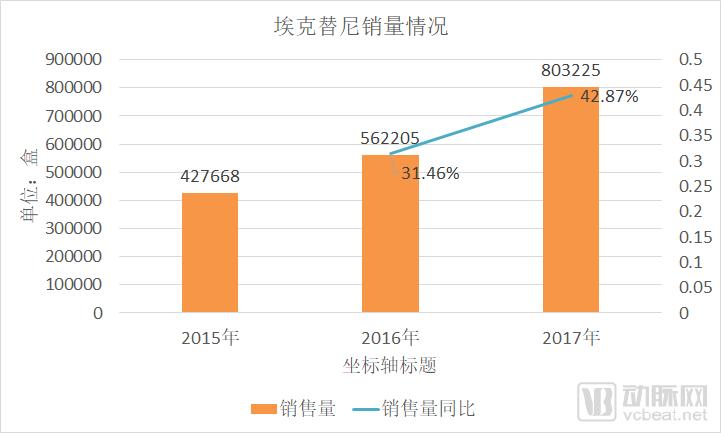
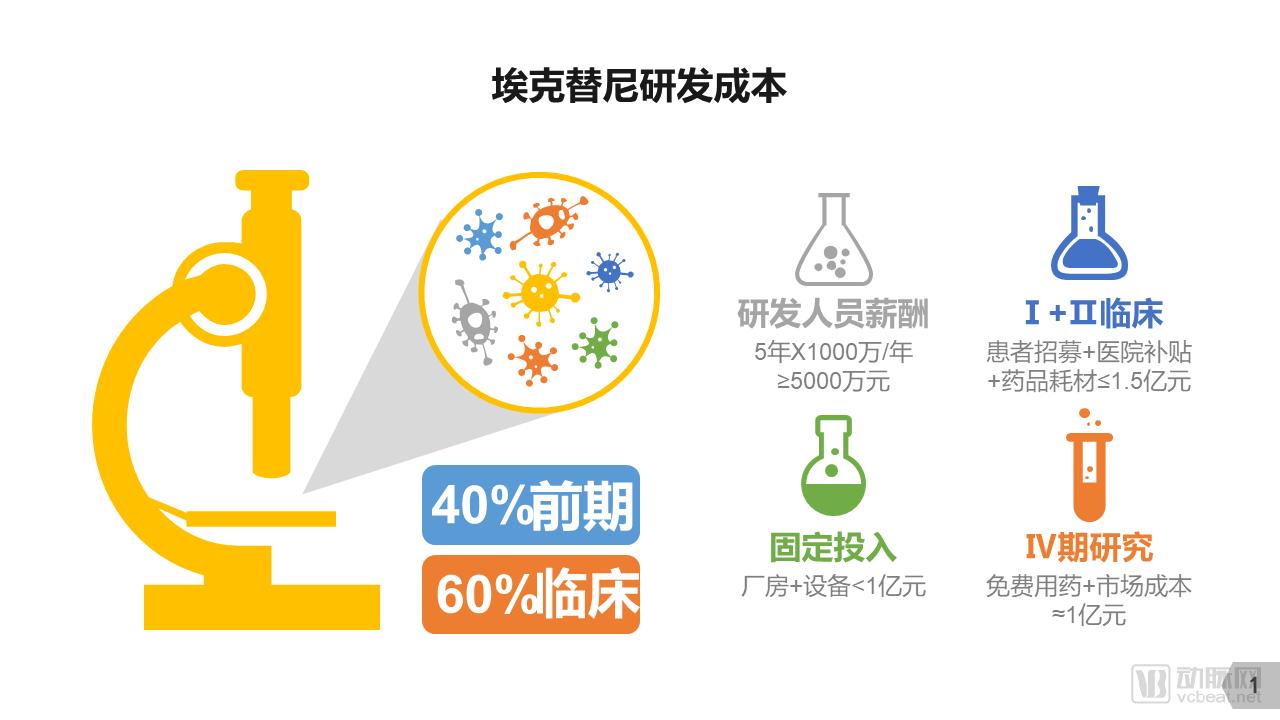
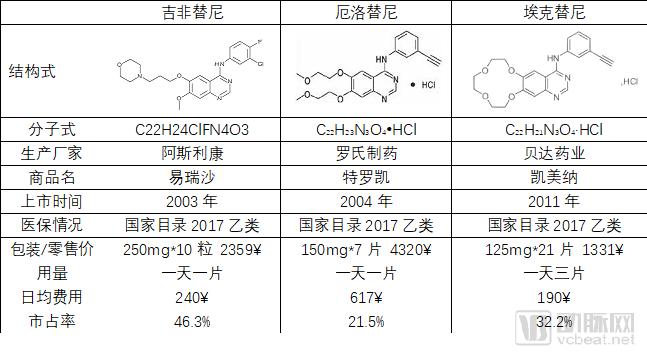
The discussion and reflection on the medical system triggered by the film "I am not a drug god" continues, especially in innovative medicines, focusing on why new drugs are so expensive and how to improve the accessibility of new drugs. The arterial network combs the general process of new drug development, and plans to restore the cost structure of new drug development through data + case. The main points of this paper are: 1. The research and development of new drugs is roughly divided into four stages, with an average time of more than 10 years and a success rate of less than 1/10. 2. Innovative drug research and development is an important method for pharmaceutical companies to enhance their competitiveness. The average R&D/sales revenue of head-to-head drug companies accounts for more than 20%; 3. Domestic cases reveal that domestic pharmaceutical companies already have certain innovation capabilities, and innovative drugs have a broad market; 4. In the cost structure of innovative drug research and development, clinical trials are the most “burning moneyâ€, personnel compensation and equipment costs are second, and the cost of phase IV research is also high; 5. Domestic policy from the policy to the market is stepping up “remediationâ€, and the research and development capabilities of innovative drugs are significantly improved, and the results will be gradually realized in the next few years. New drug research and development success rate is less than 1/10 The research and development of new drugs is roughly divided into four stages: the early stage of drug exploration, the pre-clinical research stage, the clinical trial stage, and the approval and listing stage. The main tasks in the early stage of drug exploration include target disease selection, target selection, selected lead compounds, and lead compound optimization. The process is uncertain. In general, R&D institutions will conduct research on the basis of their predecessors to ensure feasibility. The main tasks in the preclinical research phase include the identification of drug candidates, animal experiments, pharmacological studies, etc., which usually take 3-6 years. The main tasks in the clinical trial phase include I-III clinical trials, medical statistics and data analysis, which usually take 6-7 years to ensure complete and accurate data. After positive clinical trial results, the drug regulatory department will be approved for registration, production and marketing, and post-marketing Phase IV trials will be conducted to further verify the safety, efficacy, and adverse reactions of the drug. New drug development process Usually, it takes more than ten years for a drug to go from research and development to market. A big mistake will occur at any stage of the four stages and it will be overturned. Generally speaking, in the four stages of new drug research and development, the clinical trial phase has the highest elimination rate, of which the phase I clinical success rate is about 65%, the phase II clinical success rate is about 35%, and the phase III clinical success rate is about 20%. Calculated, the success rate in the clinical trial phase is less than 10%. In specific areas such as cancer, the success rate of new drug development is lower. High R&D investment is the only way to maintain long-term competitiveness From the perspective of global data, the investment in R&D of highly competitive multinational pharmaceutical companies is relatively high. The data shows that the top ten pharmaceutical companies in the world in research and development accounted for more than 20% of R&D/sales revenue in 2017, with a maximum of 45%. 2017 global R & D investment TOP10 pharmaceutical companies Data source: company announcement, intranet, arterial network From the domestic data, the R&D investment of pharmaceutical companies is relatively low, most of them are below 7%, and the highest is only 12.71%. In 2017, domestic R&D investment in TOP10 pharmaceutical companies Data source: company announcement, intranet, arterial network The global competitiveness of multinational pharmaceutical companies is not unrelated to their high R&D investment. Continuous R&D investment has spawned high-quality drug production and created a “blockbusterâ€. Some pharmaceutical companies can achieve a sales revenue of over 10 billion US dollars in the world, and lead the market for several years, bringing huge profits to the company. Through industrialized and standardized R&D processes, multinational pharmaceutical companies have actually turned the risk of new drug development into a “probability†event. Since there is always a 10% success rate, as long as you have been investing, you will always be able to produce high-quality innovative drugs. However, the natural aversion to risk has also led to some changes in the structure and approach of R&D investment. First, they tend to be more likely to suffer from a larger patient population, a longer treatment cycle, or a costly treatment area, such as tumors, cardiovascular and cerebrovascular, anti-infectives, etc., while turning a blind eye to the needs of some rare diseases and border diseases. In addition, it is also common to develop similar new drugs for the same target or to change the crystal form of the drug molecule to bypass the patent. Secondly, the way of contract research and development has emerged and emerged in the research and development of medicine, and is usually jointly developed in the form of “resultsâ€. The large pharmaceutical companies export their R&D standards and processes to the Contract Development Organization (CRO), transforming the fixed costs into variable costs, and agreeing on the results of joint development to achieve commercial value. Business models that provide technical or management support for new drug development are also beginning to emerge, including patient recruitment, experimental data management, and real-world research, all of which help reduce the cost of new drug development and increase the efficiency of new drug development. Of course, what is more important is the rise of generic drugs. Generic drugs eliminate the need for pre-drug discovery, clinical trials, and capital costs, and can increase the success rate. In terms of price, it is also easy to be accepted by the market. In the United States, for example, generic drugs account for 88% of prescriptions, meeting the needs of government health insurance and commercial insurance companies for high quality and low price drugs. In addition, the research direction of “new drugs for new drugs†has begun to emerge. It is also a low-cost, high-efficiency new drug research and development method by discovering new drugs for new drugs with expired drugs or several drugs. Beida Pharmaceutical, a quality sample of innovative pharmaceutical companies The above mentioned the innovative drug development process and the innovative investment of the head company, let us look at a domestic case - Beida Pharmaceutical. This pharmaceutical company has a single product and business, and it is a listed company. The data is open and can be discussed in detail. Beida Pharmaceutical was founded in 2003 by the team of Dr. Haigui. Its main product is ectinib (Kemena), which is mainly used in the treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with sensitive mutations in the epidermal growth factor receptor (EGFR) gene. Treatment can be used to treat locally advanced or metastatic non-small cell lung cancer (NSCLC) after failure to receive at least one chemotherapy regimen. Previous chemotherapy primarily refers to platinum-based combination chemotherapy. Ectinini was approved for listing in 2011 and achieved very good market performance. Sales revenue in 2013, 2014 and 2015 was 4.75, 7.03 and 913 million respectively. In 2016, sales exceeded 1 billion. Yuan, sales exceeded 550,000 boxes. Beida Pharmaceutical 2013-2017 Revenue Structure Data source: company annual report, arterial network In November 2016, Beida Pharmaceuticals knocked on the GEM, and Kemena contributed more than 98% of its sales revenue, and its gross profit margin contributed over 99%. It can be understood that a drug from Kemena boosted the listing of Beida Pharmaceutical. Beida Pharmaceutical's 2013-2017 net profit data Data source: company annual report, arterial network In February 2017, Ekentini entered the national medical insurance catalogue. After the price adjustment, the sales volume increased by 42.87%, basically making up for the impact of price cuts, achieving “price-for-value†and achieving sales of 1.026 billion for the whole year. yuan. In fact, before entering the national medical insurance catalogue, Kamena has been included in the scope of medical insurance reimbursement in some places, and the price has been adjusted. Eckinib sales in the past three years Data source: company annual report, arterial network The 2017 annual report also revealed that since the listing of Ektorini, the cumulative sales revenue has reached 4.518 billion yuan, and a total of 155,000 patients have taken ectinib. In the first quarter of 2018, Ekentini sales increased by more than 20% year-on-year. With the follow-up of medical insurance in various provinces, Ekentini is expected to continue to increase its volume. The most clinical trials are "burning money", and the free medicine is a big head. As the first listed company in the A-share market with innovative drugs as its main business, Beida Pharmaceutical is a high-quality sample of domestic innovative pharmaceutical companies. As a listed company, its financial disclosure is also more detailed and accurate, so we can analyze the cost structure of new drug research and development from Beida Pharmaceutical's prospectus, annual report, approval and other materials. According to data from the Drug Review Center, ectinib hydrochloride tablets (Kemena) were applied for clinical practice in December 2005 and approved in June 2006. Clinical trial data center data show that the clinical study of ectatinib hydrochloride tablets in patients with multiple tumors, especially non-small cell lung cancer patients, in Phase I and IIA in November 2007 was awarded the Ethics Committee of the First Affiliated Hospital of Zhejiang University Medical College in November 2007. Approved, the experiment was designed using a non-randomized controlled trial. In this experiment, a total of 71 people from multiple groups participated in the experiment. The trial ended in December 2009. In the same period, the Phase I study of ectinib hydrochloride tablets was also conducted in Peking Union Medical College Hospital from August 2007 to July 2008. A total of 16 people from two groups participated in the trial. Ectinini applied for production in August 2010 and was approved in June 2011. That is to say, the data of the first two clinical trials performed well and had the conditions for approval. Industry insiders estimate that according to the industry's prevailing clinical patient recruitment, hospital subsidies, drug supplies and other costs, the clinical trial cost of ectinib before listing is about 150 million yuan. In addition to the cost of clinical trials, the cost of drug research and development also includes R & D personnel compensation, preliminary research, equipment purchase, plant construction, etc., which can be found in the prospectus. According to the prospectus, the fixed assets of Beida Pharmaceutical at the end of 2013 were 87.79 million yuan, and the end of 2014 was 86.27 million yuan. Considering the input of fixed assets on behalf of equipment and workshops, it is a one-time investment. 100 million yuan. In 2013 and 2014, Beida Pharmaceuticals paid 11.3 million yuan and 13.88 million yuan. Based on the five-year research and development cycle, the accumulated R&D manpower expenditure was about 50 million yuan. Therefore, it can be judged that the total research and development cost of ectinib is about 2.5-300 million yuan. Among them, clinical research is the most "burning money." After the introduction of ectinib, a free drug use program was also included as part of the Phase IV study. In conjunction with the Phase IV clinical study, Beida Pharmaceutical and the China Pharmaceutical Industry Research and Development Association jointly launched the Eckinib follow-up free drug project. . After 6 months of continuous use of ectatinib at the expense of the patient, the registered doctor of the designated medical center of the study evaluated the patient with effective efficacy according to the RECIST criteria and obtained the Eckinib follow-up free drug project office for review. Subsequent free medication for Tini. The data shows that the cumulative number of people who entered the free drug use was 50,440, and the cumulative drug delivery was 2,744,281 boxes. This data is more than the sales of ectinib in the same period, and can also be regarded as an important cost. According to the prospectus, from 2013 to January-June 2016, the cost of free clinical use of ectinib in phase IV was 8.734 million yuan, 16.822,000 yuan, 25.816 million yuan and 16.24.64 million yuan. All of them have been expensed in the current period. Based on this calculation, since the listing, the total cost of the ectopini free drug use project is about 100 million yuan, but this is not completely calculated based on the experimental cost of the IV phase. The marketing is also an important purpose, the difference is the accounting treatment. . It is worth noting that erfitinib (Iressa) and erlotinib (Troquet) have similar promotion projects, and AstraZeneca and China Charity Federation have established Yirui. The Sha Charity Drugs Program was officially launched in 2007; Roche and the China Charity Federation launched the Tarceva Charity Drugs Program, which was launched in 2008. Of course, the example of ectinib does not fully represent the R&D costs and R&D structure of innovative drugs. First of all, its concept of medicine is the "me too" innovative drug for gefitinib and erlotinib. It is not entirely necessary to find a target and to screen compounds from scratch, so the cost will be relatively low. In addition, considering the domestic labor costs and resource costs, the total cost of ectinib should be relatively low. However, from the case of Beida Pharmaceutical, the main cost and secondary cost of innovative drug research and development, as well as the cost structure, can be roughly seen, which provides a benchmark value for new drug investment. Comparison of gefitinib, erlotinib and ectinib Data source: medication reference, drug network, 1 drug network, arterial network Solve the "drug god" problem: perfect policy, industry follow-up, capital boost "I am not a drug god" has exposed the current situation of imbalanced supply and demand of innovative drugs in China. Most of the innovative drugs are developed by multinational pharmaceutical companies. They enjoy full pricing power. Even if they implement uniform pricing globally, there will be no small burden for Chinese patients with high incomes without developed countries, so patients will turn. Cheap Indian generics. Why India can produce low-cost generic drugs, which has nothing to do with its medical policy environment. The most important one is the compulsory licensing of patents. That is, the Indian government allows domestic companies to carry out imitation before the original drug manufacturers’ patents have expired. The purpose is to protect the national drug, so India has become a "forbidden zone" for patented drugs. However, due to factors such as the patent protection system and trade rules, although China has this law, it has never been implemented. There are three ways to solve the problem of “drug godâ€. The first is to enhance our own innovative drug research and development capabilities, to provide better innovative drug products with the cost advantage and competitive advantage of local enterprises. The second is to innovate multinational pharmaceutical companies. The price of medicines has dropped, including tariffs, value-added taxes, price negotiations, etc. The third is to improve the social security system, such as medical insurance for major illness relief, commercial insurance, and charity donation. In fact, these three aspects are indeed what we are doing. For example, the reform of drug trials, from “resolving the backlog†to “encouraging innovationâ€, innovative drugs can enter the green channel and give priority to review and approval; for example, encourage the innovation of pharmaceutical medical devices, including the implementation of the MAH system, and the clinical trial institutions to the filing system. International multi-center clinical trial data recognition, drug patent link system, encourage CRO, CMO development and other policies and measures to provide supporting support for the development of innovative drugs. In terms of reducing the price of innovative drugs, including medical insurance negotiations, “price-for-valueâ€; at the same time, China has implemented “zero tariffs†on anti-cancer drugs since May 1; on June 20, the State Council executive meeting has confirmed that it has accelerated its listing abroad. New drug approval, implementation of anti-cancer drug price reduction measures, and strengthening the supply of shortage medicines and other measures. The policy is perfect, the industry is following up, and the leading enterprises represented by Hengrui Medicine, Fosun Pharma, Shijiazhuang Pharmaceutical Co., Kelun Pharmaceutical, Tasly Pharmaceutical, and Beida Pharmaceutical are actively deploying innovative drugs. The data of the drug testing center shows that Nearly 80 innovative drugs have entered the phase III clinical or production declaration stage, covering areas such as anti-tumor, respiratory system and diabetes, including the most advanced pd-1/pd-1 drugs. At the capital level, since 2013/2014, VC/PE capital has entered the field of innovative medicine and entered a period of continuous growth. Arterial network data show that since 2014, there have been 323 financings in the domestic new drug field, involving an amount of about 32 billion yuan, and the average single financing amount is close to 100 million yuan. It also gave birth to Cinda, Mengke, Songli, Dingding, and Baekje Shenzhou. Such a head business. Perfect policies, industry follow-up, capital boost, and domestic innovative drugs are on a path of sustained growth. In the future, there will be fewer and fewer alternative heroes like "Pharmaceutical God". Antitumor And Chemotherapy Antiemetic Antitumor And Chemotherapy Antiemetic,Tropisetron Hydrochloride Tablets,Tablets Of Tropisetron Hydrochloride,Tablets For Antitumor And Antiemetic Shandong Qidu Pharmaceutical Co., Ltd. , https://www.qdyypharma.com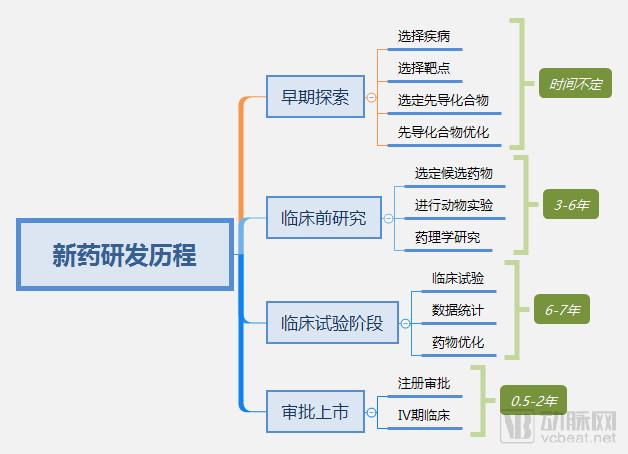
Innovative drug research and development of ten billion dollars, the results?