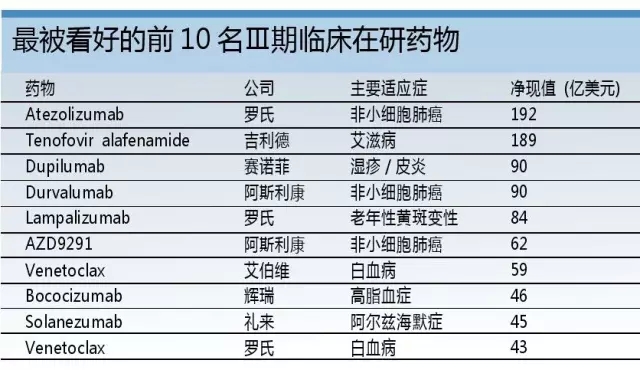
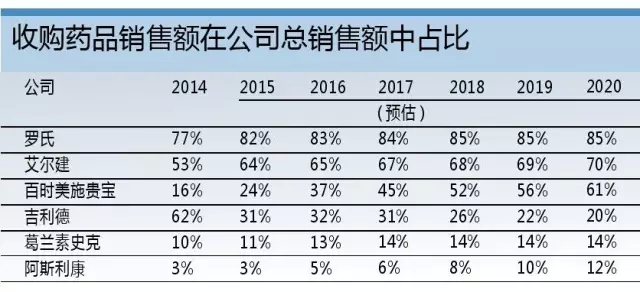
Release date: 2015-10-10 The growing dependence of large pharmaceutical companies on mergers and acquisitions has been questioned, but this development path is now officially recognized: an analysis using EvaluatePharma data covering the top 20 global pharmaceutical companies indicates that these companies are expected to be acquired through mergers and acquisitions in 2016. The sales of the products will be equal to the sales of its internally developed products. The analysis showed that 64% of the top 100 Phase III clinical drugs measured by net present value were derived from external acquisitions. Among them was Roche's anti-PD-L1 monoclonal antibody, atezolizumab, which was acquired by the acquisition of Genentech. Roche knows best about "exciting external forces" The analysis showed that Roche was a company that relied heavily on mergers and acquisitions. Another phase III clinical drug candidate, lampalizumab, was also acquired through mergers and acquisitions from Tanox, which was acquired by Genentech. In 2014, 77% of Roche's sales came from acquired drugs, which is expected to reach 85% in 2020. Such a strategy seems to be no harm to the pharmaceutical giant: the company has maintained its third position in the pharmaceutical industry for nearly five years. Allergan also hopes to achieve similar growth in the introduction of innovative drugs, which is not surprising given the volume of transactions it has achieved in recent years. Aerjian's trading spree should help it become the world's 9th largest pharmaceutical company by 2020, and the company ranked 19th in 2014. At the same time, Bristol-Myers Squibb is expected to make the biggest leap in outsourcing: last year only 16% of its sales came from acquisitions, but this number will rise to 61% in 2020. This is mainly due to its PD-L1 inhibitor Opdivo, which is expected to be the biggest growth driver for Bristol-Myers Squibb, with sales expected to reach $7.9 billion in 2020. In 2009, Bristol-Myers Squibb acquired Opdivo and another heavy drug Yervoy through the acquisition of Medarex, and the $2.4 billion advance payment seemed to be worthwhile. Bristol-Myers Squibb's second major anti-coagulant drug, Eliquis, from 2020 was also from outside the company, which was originally developed by DuPont Pharmaceuticals. Gilead relies on "self-reliance" However, there are also some companies whose trends are contrary to the above trends. For example, Gilead Science's sales from externally developed drugs will decline. The company's most promising phase III clinical competition product, the AIDS drug Tenofoviralafenamide, was developed by itself. The drug is a prodrug of Gilead's old AIDS treatment Viread. The potential of Tenofoviralafenamide is dwarfed by Gilead's portfolio of hepatitis C drugs, which combines its in-house R&D with external acquisitions and is expected to remain Gilead's best-selling drug by 2020. This is followed by GS-9857/SOF/GS-5816 triple hepatitis C therapy, which is currently in Phase II clinical stage. However, this may change soon. If not unexpected, Gilead will use its recent $10 billion debt ceiling to trade the risk, extending the scope beyond the scope of hepatitis C treatment. GlaxoSmithKline and AstraZeneca also have no M&A activity – the former has avoided mergers and acquisitions, and the latter has not achieved much through its Medimmune acquisition. For the two British pharmaceutical companies, the strategy of relying on authorized introduction of products is currently more complicated, at least for AstraZeneca. It has been criticized that the company is paying too much for risky, early-testing assets, such as Rigel Pharmaceuticals' rheumatoid arthritis candidate Fostamatinib, which AstraZeneca eventually returned after Phase III clinical trials. . However, AstraZeneca's internal R&D efforts have achieved some success and made the cooperation project more favorable for it – especially with the development of the anti-PD-L1 monoclonal antibody Duvalumab by Celgene, which was earned in advance. $450 million in advance payment. The project's assets are critical to AstraZeneca's future success and will be its most promising Phase III clinical competitive product. At the same time, GlaxoSmithKline expects sales to remain stable between now and 2020 as the company focuses on its stable but not exciting vaccine and consumer healthcare business. Perhaps, the company should follow other pharmaceutical companies and acquire some innovative drugs to support its development prospects. Source: Pharmaceutical Economics Frozen Butterfly Vannamei shrimp frozen butterfly vannamei shrimp Butterfly Vannamei Shrimp,Frozen Butterfly Vannamei Shrimp,Fresh Butterfly Vannamei Shrimp,Fresh Frozen Butterfly Vannamei Shrimp Zhoushan Junwei Aquatic Products Co., Ltd. , https://www.junweiaquatic-intl.com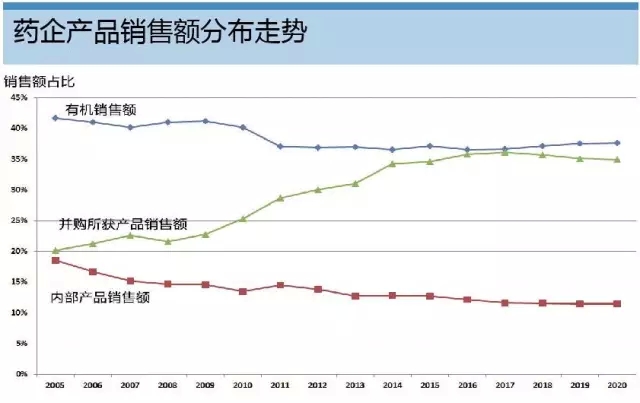
Data talk: "buy buy and buy" into a pharmaceutical company growth shortcut